The Process of Creating a New Drug: Clinical Trials
Written by Ivory Chen
All drugs require an undergoing of intensive testing and trials before they are released for public use. There are multiple phases that the newly developed treatment must pass: pre-clinical testing, and 4 separate phases of clinical trialing. The endless testing guarantees both the safety and effectiveness of the drug.
Prior to clinical trialing, a drug must be tested outside of the patient’s body, in a lab. For example, in the case of gene-editing, DNA would be extracted from a patient’s body to be tested on. After the treatment is applied, the DNA is amplified and observed to determine if the treatment was effective, on a small scale. Once this step is guaranteed, the drug can move into clinical testing.
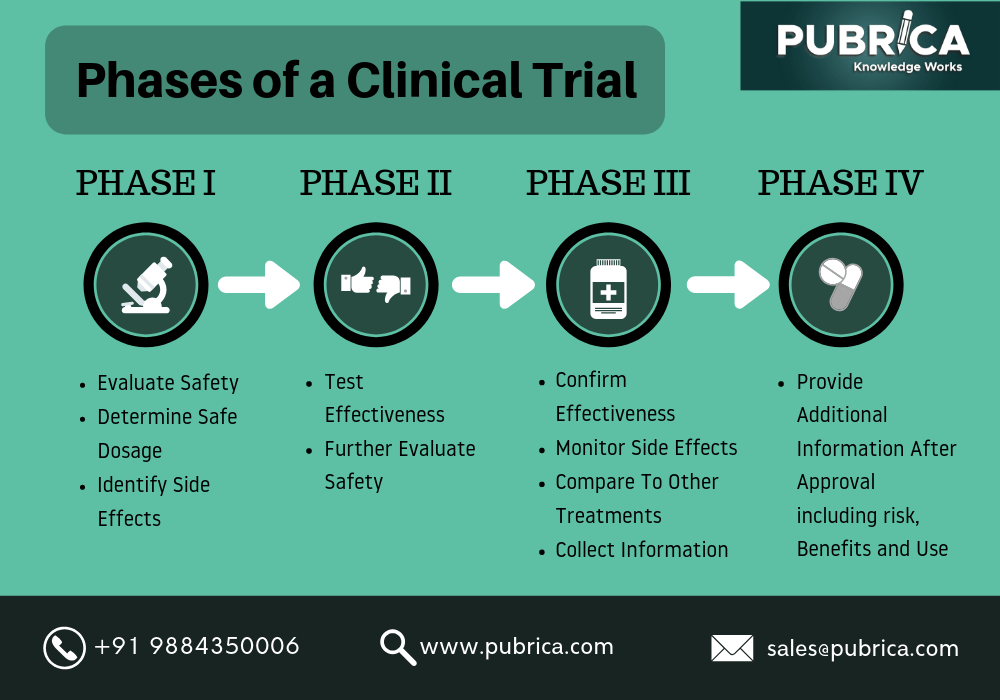
Phase I of a clinical trial ultimately determines the safe dosage of the drug and the general safeness of the drug when applied to humans (small-scale testing ~10-20 people). Phase II takes the testing up to a larger scale, looking specifically at the effectiveness of the drug. Next, Phase III looks at the drug comparatively. In comparison to other present treatments, does this drug make a breakthrough or provide a different outcome that isn’t achievable by other treatments? Finally, Phase IV involves gaining FDA approval for the drug, and continuing to observe long-term effects of the drug. These processes all guarantee the safeness of marketable treatments in America.
References
“Clinical Trials.” World Health Organization, World Health Organization,
www.who.int/health-topics/clinical-trials#tab=tab_1. Accessed 20 July 2024.
Written by Ivory Chen from MEDILOQUY